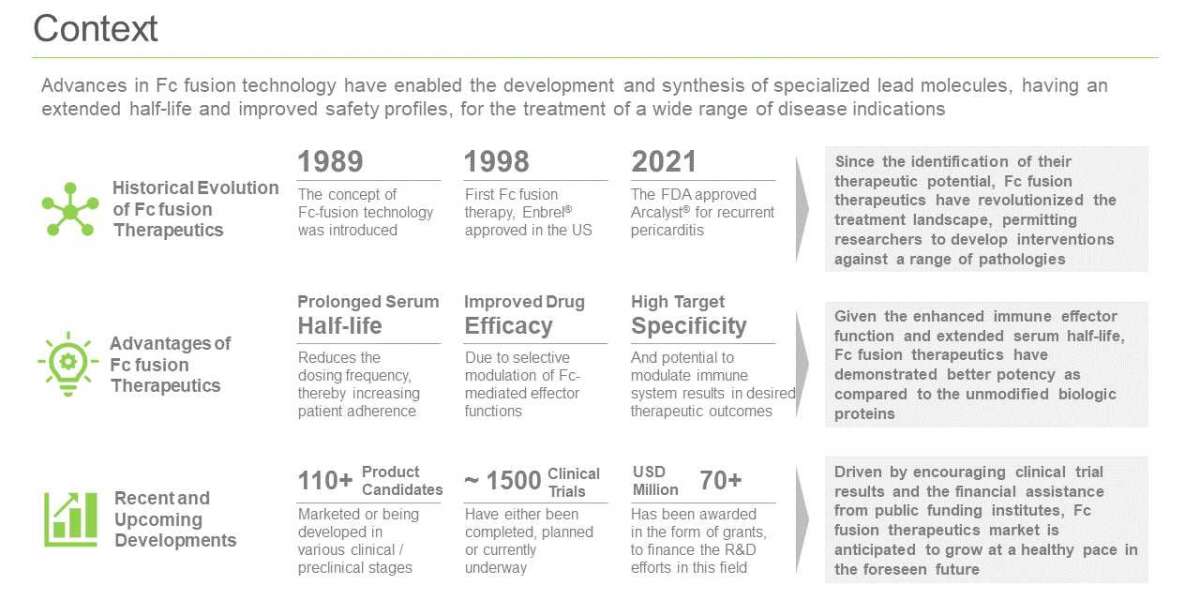
Since the approval of Enbrel® in 1998 for the treatment of rheumatoid arthritis, Fc fusion therapies have evolved into a prominent class of therapeutics; currently, several therapies are being developed for a variety of disease indications
London
Roots Analysis has announced the addition of “Fc Fusion Therapeutics Market, 2021-2030” report to its list of offerings.
With 13 drugs approved in the European Union and the US, Fc fusion therapeutics are considered to be one of the most successful classes of IgG-based products. The success of these biopharmaceutical products can be attributed to their diverse biological and pharmacological properties, including an extended serum half-life, enhanced Fc mediated effector functions, easy expression, increased stability and aggregation resistance, modulated immunogenicity and improved safety profiles, for the treatment of a wide range of disease indications
To order this 130+ page report, which features 95+ figures and 140+ tables, please visit https://www.rootsanalysis.com/reports/fc-fusion-therapeutics-market.html
Key Market Insights
Presently, more than 115 Fc fusion therapeutics are approved / under development
Over 60% of the aforementioned candidates are being evaluated in clinical stages; of these, 50 molecules are in advanced phases (Phase II and above) of clinical trials. This is followed by more than 30% of the Fc fusion therapeutics which are already marketed for various disease indications. It is worth noting that majority of the pipeline therapies (18) are being developed to target PD-L1 for the treatment of oncological disorders.
More than 25 companies claim to be engaged in the development of Fc fusion therapeutics
Post 2001, there has been a significant rise in the number of companies working in this domain. It is worth noting that majority (48%) of the firms engaged in this domain are based in North America, followed by those headquartered in Asia-Pacific (38%) and Europe (3%).
Over 1,450 clinical trials have been registered for the evaluation of Fc fusion therapeutics, globally
Of these, most of the trials were / are being conducted in North America (48%). Within this region, more than 50% of the total patients were enrolled in trials focused on the evaluation of these therapeutics. This was followed by studies being conducted in Europe and Asia Pacific.
Close to 200 grants have been awarded to support research on Fc fusion therapeutics, since 2010
Around USD 68 million have been awarded to various organizations working in this domain, since 2010. Further, almost 80% of the total grants were awarded for a support period of 1-5 years.
Over 10,000 patents have been filed / granted related to Fc fusion therapeutics, since 2018
Of these, majority (45%) of the patents were filed / granted in North America, followed by Asia-Pacific (21%). Moreover, in addition to the industry players, various patents related to Fc fusion therapeutics were filed by academic institutes as well.
Partnership activity within this domain has grown significantly between 2015 and 2021
The maximum number of partnerships for Fc fusion therapeutics were signed in 2020. Further, product development and commercialization (32%) emerged as the most popular type of partnership model adopted by stakeholders in this domain. This was followed by product development agreements (26%), acquisitions (21%), and manufacturing and supply agreements (11%).
Funding and Investments within this domain has shown a significant growth between 2016 and 2021
Around USD 450 million was raised in the Fc fusion therapeutics domain, through various funding rounds. It is worth mentioning that majority of the amount (~USD 550 million) was raised through venture capital financing.
At present, therapies targeting ophthalmological disorders represent majority of the market share (in terms of sales revenue)
We believe that, in the foreseen future the trend is likely to remain the same. The market opportunity within this therapeutic area is likely to be distributed among indications, such as ophthalmological disorders, and hematological diseases. This is followed by genetic disorders which represent over 30% of the market share.
To request a sample copy / brochure of this report, please visit this link
Key Questions Answered
- Who are the leading players involved in the development of Fc fusion therapeutics?
- Which geographies are the most active in conducting clinical trials on Fc fusion therapeutics?
- Which are the leading funding organizations providing grants for Fc fusion therapeutics?
- Which partnership models are commonly adopted by industry stakeholders engaged in the Fc fusion therapeutics market?
- How is the current and future market opportunity likely to be distributed across key market segments?
The financial opportunity within the Fc fusion therapeutics market has been analyzed across the following segments:
- Type of Target Indication
- Neutropenia
- Graft Versus Host Disease
- Breast Cancer
- Rheumatoid Arthritis
- Non- Small Cell Lung Cancer
- Neovascular (wet) Age-related Macular Degeneration (AMD)
- Hemophilia A
- Neuromyelitis Optica Spectrum Disorders
- Systemic Lupus Erythematosus
- Type of Fusion Molecule
- Antibody
- Cytokine
- Growth Factor
- Receptor ECD
- Others
- Type of Therapy
- Combination
- Monotherapy
- Route of Administration (RoA)
- Subcutaneous
- Intravenous
- Intravitreal
- Geographical Regions
- North America
- Europe
- Asia- Pacific
- Latin America
- Middle East
- Rest of the World
The research includes detailed profiles of key players (listed below); each profile features an overview of the company, its financial information (if available), a description of the services offered, details on recent developments and an informed future outlook.
- Alphamab Oncology
- Amgen
- Acceleron Pharmaceuticals
- Bristol-Myers Squibb
- Sanofi
For additional details, please visit
https://www.rootsanalysis.com/reports/fc-fusion-therapeutics-market.html
or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Regulatory T-Cell Therapies (Tregs) Market, 2021–2035
- CD47 Targeting Therapeutics Market, 2021-2035
- Light Activated Therapies Market, 2021-2030
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com